Pharmanza Herbal
Research and Development (R&D) Unit
Pharmanza Herbal has dedicated state-of-the-art R&D facilities run by esteemed research faculty members. The R&D unit primarily focuses on developing standardised herbal extracts and conducts assessments of their physicochemical stability, pharmacokinetics, and clinical studies.
100+
Research Publications
The R&D unit comprises distinct facilities that promote pharmacognosy, botanical studies, and analytical research.
Pharmacognosy and Botany Centre (PBC)
The PBC offers research in the Botany of Medicinal Plants, including their nomenclature, authentication, taxonomy, microscopic and macroscopic analysis, pharmacognosy, and the identification of plant adulteration.
Research activities are covered by various in-house supported projects. The Pharmacognosy Section primarily focuses on microscopic plant analysis (anatomical studies). Similarly, the Botany Section maintains a herbarium of selected medicinal plants and provides instrumentation facilities for the study of plant morphology and anatomy, ensuring the authentication of botanicals at Pharmanza Herbal and publishing peer research on botanical identity to avoid adulteration. The PBC actively engages in raw material analysis, validation, and its authentic and sustainable supply.


New Product and Process Development (NPPD)
The NPPD in R&D is a dynamic and innovative team responsible for driving business growth through the development of novel products and processes. This department leverages cutting- edge technologies, industry trends, and customer insights to design, prototype, and test new products and processes that meet customer needs and exceed market expectations. By fostering a culture of innovation and collaboration, this department enables the company to stay ahead of the competition, expand into new markets, and enhance its product portfolio.
Through rigorous testing, validation, and scaling, the New Product and Process Development Department ensures seamless transition of new products and processes to commercialization, delivering significant value to customers and stakeholders alike.
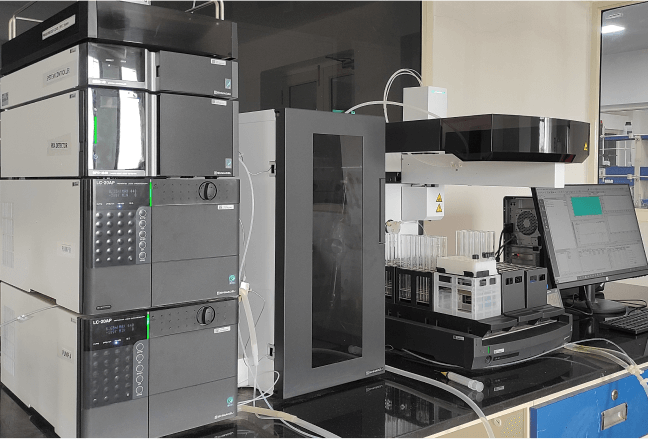
Analytical Development and Instrumentation Centre (ADIC)
The ADIC plays a crucial role in botanical product development and standardisation. It conducts analytical method development and validation in a state-of-the-art laboratory equipped with instruments such as HPLC-PDA, LC-MS/MS, and HPTLC.
Highly sensitive and validated analytical methods, based on HPLC-PDA, are employed to quantify bioactive compounds. Comparative HPTLC/HPLC fingerprinting, as well as hyphenated approaches like HPTLC-MS and LC-MS/MS, are utilised for characterising substances, identifying unknown components, and detecting potential adulterations. Robust analytical methods are transferred to the quality department to ensure that products consistently meet expected quality standards throughout their lifecycle.